Abstract
Introduction:
Previous population-based studies in pediatric acute lymphoid leukemia (ALL) have identified racial and ethnic differences in incident childhood ALL (Med Pediatr Oncol 2002;39:554), but the degree to which these disparities persist among adult ALL patients, who have distinct differences in the molecular subtypes of ALL compared to children, is less clear. Larger studies that look across broad populations of patients are needed to assess these findings in adult ALL. We therefore used the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database to characterize the incidence of distinct subtypes of ALL in adults, to evaluate whether differences in racial or ethnic incidence rates are seen in the adult population and relate to molecular subtypes of disease.
Methods:
The 1973-2014 SEER-18 database was used to identify adults, age 20 years or older, diagnosed with lymphoid leukemia between 2000 and 2014. We included only patients with documented race/ethnicity and known age at diagnosis. Leukemia subtypes were defined by the third edition of the Internal Classification of Diseases for Oncology (ICD-O-3) and stratified by cell lineage and BCR-ABL mutation status. BCR-ABL mutation status was added to SEER in 2010 so analysis of these subgroups includes only patients diagnosed 2010-2014. Analysis was carried out comparing ethnicity, Hispanic (H) vs. non- Hispanic (NH), as well as race/ethnicity, non-Hispanic White (NHW) vs. Hispanic White (HW), non-Hispanic Black (NHB) or non-Hispanic Asian-Pacific Islander (NHAPI). Age-adjusted incidence rates (IRs) per 100,000 person-years were calculated using the Rate Session in SEER*Stat. Incidence rate ratios (IRR) were calculated using STATA software. Two-sided p-values were calculated using the mid-p test.
Results:
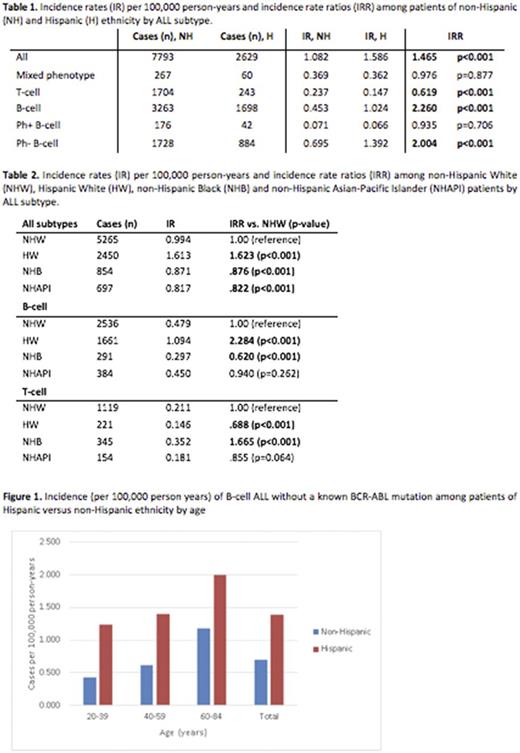
An overall higher incidence of ALL was present among patients of Hispanic ethnicity as compared to non-Hispanic (IR 1.59 vs. 1.08, IRR 1.47 p<0.001). The highest rates of ALL occurred in HW versus NHW, NHB, NHAPI (IR 1.613 HW vs. 0.994 NHW, IRR 1.623 p<0.001). The lowest rates occurred in NHB and NHAPI (IR 0.871 NHB and 0.817 NHAPI, IRR .876 and .822 respectively vs. NHW, p<0.001). The higher incident ALL rate among HW appeared to be explained by rates of B-ALL (IR 1.094 HW vs. 0.479 NHW, IRR 2.284 p <0.001). Further exploration, based on molecular subtype, revealed that there was no difference in incidence of BCR-ABL mutated B-cell ALL based on ethnicity (IR 0.066 H vs. 0.071 NH, IRR 0.935 p=0.706). There was, however, an increased incidence of B-cell ALL without a reported BCR-ABL mutation among patients of Hispanic ethnicity (IR 1.392 H vs. 0.695 NH, IRR 2.004 p<0.001). T-ALL was least prevalent among HW and there was no difference in incidence of mixed phenotype leukemia. NHB patients has significantly lower rates of ALL overall, however they had a higher age-adjusted incidence of T-cell ALL (IR 0.352 NHB vs. 0.211 NHW, IRR 1.665 p<0.001).
Conclusions:
In children, published data suggests an overall increased incidence of ALL among patients of Hispanic ethnicity and a decreased incidence among patients of Black race. Our analysis of SEER data from 2000-2014 shows that these patterns persist among adult patients. Interestingly, in this large, population-based study, we found that the higher rates of ALL among Hispanic populations are largely related to a higher incidence of Ph-negative B-cell ALL, a pattern that persists across all ages (figure 1). We also found an overall decreased rate among NHB patients, despite an increase in T-cell ALL. This is also consistent with findings seen in childhood ALL. The specific trends with Ph-negative B-cell ALL have not been previously described, likely due to limited availability of molecular data until this point. In this study, we found no difference in incidence rates of Ph-positive ALL in Hispanic vs. non-Hispanic populations across all age groupings. Our findings suggest that some underlying mechanism may contribute to higher rates of Ph-negative ALL in this population. Previously hypothesized explanations for this difference include susceptibility genes (ARID5B, PIP4K2A) which may be enriched in certain populations, environmental toxins or chemicals, viruses or other environmental factors. Whether some or most of these factors would account for the approximately double incidence of Ph-negative B-cell ALL across all ages remains unclear and requires further study.
Fathi: Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Medimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Pfizer: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Brunner: Celgene: Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal